Covid-19 treatment with combination of antiviral drugs as an Aerosol or I-V mode
- (0)
Canadian Armenian Dr. Sarkis Yeretsian recently sent a proposed Covid-19 treatment with combination of antiviral drugs to MOH in Yerevan.
The FDA approved has proposal and they expressed their readiness to fund a trial based on my proposal. He sent his proposal to both Universities MvGill and Montreal.
Dr. Yeretsian’s approach has special features one of them is the usage of Human organe on chip technology.
***
By Sarkis Yeretsian, M.D. FRCSC.
Introduction:
History of the COVID-19 Pandemic 2019-2020
- The first cases of atypical pneumonia in Wuhan were detected in November 2019. Chinese officials reported them to the WHO’s China Country Office in Beijing only on December 31.
- On January 4, 2020 the WHO tweeted that China had reported cases of pneumonia with no death in Wuhan, Hubei Province.
- On January 5, the agency described a pneumonia of unknown cause.
- By January 7, Chinese scientists had identified the causative agent as a novel type of coronavirus. Its genetic sequence was transmitted on January 12 to WHO.
- China shared the genetic sequence of the virus on January 12.
- Among 41 cases the earliest symptoms began on December 8.
- On January 12, the WHO reported that there was no clear evidence human-to-human transmission.
- Chinese authorities claimed ‘’No additional cases have been detected since 3 January 2020.”
- On January 14 human-to-human transmission was confirmed by Jasper Fuk-Woo Chan and colleagues published in The Lancet.
- Chinese experts from Beijing visited Wuhan to investigate the outbreak and were followed by a team from WHO on January 20-21.
- The International Health Regulations (IHR) Emergency Committee met on January 22-23. After a split vote they reconvened on January 30 and concluded the outbreak fulfilled criteria for a Public Health Emergency of International Concern (PHEIC) which Dr. Tadros declared the same day.
- It took 4 days to inform the world about the existence of this new atypical pneumonia. It took just 30 days to declare a PHEIC.
- Since November 2019, Wuhan, China the outbreak of Coronavirus 2 (SARS-CoV-2) infection has had exponential spread through Asia, Europe, USA, Canada and the whole world causing severe acute respiratory syndrome. Despite the acquired experience in the pandemic of SRAS, Ebola, MERS the world health organizations were unable to stop the spread of this novel coronavirus.
- The scientists from across the world are working desperately to find new treatments. They have been exploring treatments such as hyperimmune globulin, antiviral drugs, hydroxychloroquine, Remdesivir, immunosuppressive, vaccine. It is crucial to discover a new medical treatment, in a short period of time, until an effective vaccine could be used safely.
Proposed Method:
Effectiveness of the proposed antiviral multidrug therapy as Aerosol or IV mode depends on the availability of human organs-on-chips technology before any clinical trial.
Ribavirin has already been used as an Aerosol in the treatment of viral pneumonia.
Salbutamol could be added to the combination of antiviral drugs.
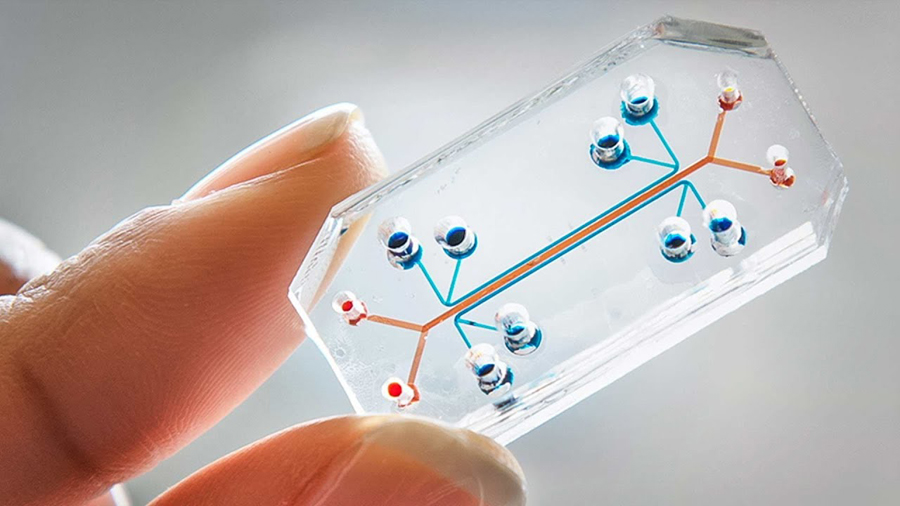
What is organ-on-chip system?
Organs-on-chips are micro-engineered biomimetic systems containing microfluidic channels lined by living human cells, which replicate key functional units of living organs to reconstitute integrated human organ-level pathophysiology in vitro. These microdevices can be used to create test efficacy and toxicity of drugs and chemicals, and in vitro models of human disease.
They potentially represent low cost-cost alternative to conventional animal models for pharmaceutical, chemical and environmental applications.
Microengineering is used to fabricate a multilayered microfluidic device that contains two parallel elastomeric microchannels separated by a thin porous membrane, along with two full-height hallow vacuum chambers on either side; this require 3.5 days to complete. For example, to create breathing lung-on-chip that mimics the mechanically active alveolar-capillary interface of the living lung, human alveolar epithelial cells and microvascular endothelial cells are cultured in the microdevice with physiological flow and cystic suction applied to the side chambers to reproduce breathing movements.
This protocol can be adapted to develop many human organs on chip: heart-on-chip, kidney-on-chip, vessel-on-chip, skin-on-chip, brain-on-chip.
Human-on-a-chip: Researchers are working towards building multi-channel 3D microfluidic cell culture system that compartmentalizes microenvironments in which 3D cellular aggregates are cultured to mimic multiple organs in the body. Most organ-on-chip models today only culture one cell type, so even though they may be valid models for studying whole organ functions, the systemic effect of a drug on the human body is not verified.
Replacing animal testing: In the early phase of drug development animal models were the only way of obtaining in vivo data that would predict the human pharmacokinetic responses. Experiments on animals are lengthy, expensive, controversial and creates ethical problems. Animal models are often subjected to mechanical or chemical techniques that simulate human injuries. There are also concerns to the validity of such animal models due to the deficiency in cross-species extrapolation.
Therefore, mimicking a human’s physiological responses in an in vitro model needs to be made more affordable and needs to offer cellular level of control in biological experiments: biomimetic microfluidic systems could replace animal testing.
Scientific research works performed in Canadian institutions and laboratories
List of all COVID-19 clinical trials authorized by Health Canada
There are 19 Canadian institutions and laboratories working to discover of novel therapies in the treatment of COVID-19. The results of clinical trial could take many months or even years before we reach a conclusive outcome. We need fast drug testing in the laboratory without animal experimentation using Human-Organ-on-Chip system.
It is proposed to tap into the acquired 40 years of experience in the successful triple viral treatment of AIDS to combat COVID-19 or recent progress achieved in the viral treatment of Hepatitis C.
- Confirmation of multiple antiviral drugs in the treatment of COVID-19 in the laboratory using Organ-On-Chip technology specially on intubated patients by performing a biopsy of pulmonary infected tissue.
- Once the effectiveness of combination of drugs have been consolidated a clinical trial could be performed;
- Proposal in the treatment of COVID-19 based on evidence supported by pharmacological knowledge as well as on microfluidic technology;
- Combination of multi-viral drugs could be used as an aerosol or I-V mode.
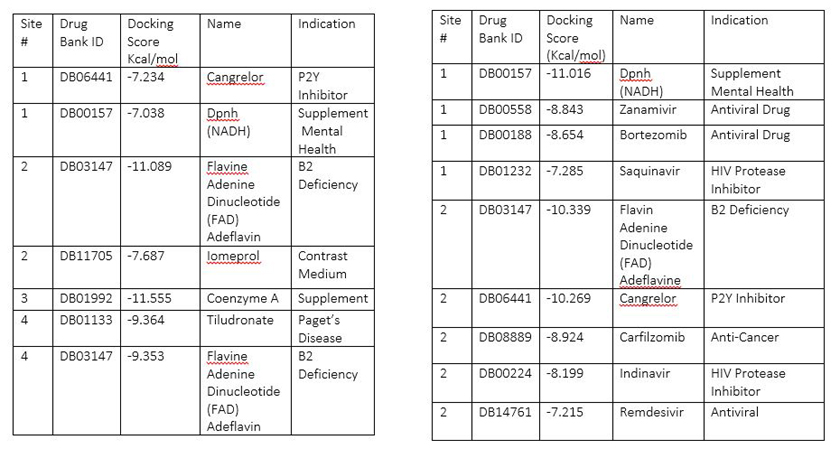
A search for medications to treat COVID-19 via in SILICO molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease.
Table 1: Highest scoring molecules for SARS-CoV-2 spike glycoprotein Table 2: Highest scoring molecules for SARS-CoV-2 3CL Main protease
Conclusion: Zanamivir, Indinavir, Saquinavir, and Remdesivir are among the exciting hits on the 3CL Pro main proteinase. It is also exciting to uncover that Flavin Adenine Dinucleotide (FAD) Adeflavin, B2 Deficiency medicine, Coenzyme A, a coenzyme, may also be potentially used for the treatment of SARS-Cov-2 infections. The use these off-label medications may be beneficial in the treatment of the COVID-19.
References (16)
Journal Pre- proof: A search for medications to treat COVID-19 via in silico molecular docking of the SARS-CoV-2 spike glycoprotein and 3CL protease. PII: S1477-8939(20)30115-0
JAMA: Original Investigation: Presenting Characteristics, Comorbidities, Outcomes Among 5700 Patients Hospitalized with COVID-!9 in the New York City Area.
Government of Canada, Gouvernement du Canada: Vaccines and treatments for Covid-19: List of all COVID 19 clinical trials authorized by Health Canada.
THE LANCET: Infectious Diseases: Estimates of the severity of coronavirus diseases 2019: a model-based analysis.
THE LANCET: Clinical course and risk factors for mortality of adult inpatients Covid-!9 in Wuhan, China: a retrospective cohort study.
Université de Montréal: Institut de Cardiologie de Montréal: New clinical trial for treatment of Covid-19 positive patients to start at NYU Grossman school of Medecine in partnership with Montreal Heart Institut.
THE LANCET: Offline: Why President Trump is wrong about WHO.
NEW ENGLAND JOURNAL of MEDECINE: Prevention of HIV-1Infection with Early Antiretroviral Therapy. August 11, 2011. N Engl J Med 2011; 365:493-505 DOI: 10.1056/NEJMoa1105243
THE Lancet: COVID-19: consider cytokine storm syndrome and immunosuppression. Correspondence.
National Post: Remdesivir: Accidentally released results suggest potential COVID-19 drug failed human trial.
THE LANCET: Successful treatment of HIV eliminates sexual transmission. Myron S Cohen Published: May 02, 2019. DOI: https//dou.org/10.1016s0140-6736(19)30701-9
NEWS BREAKTHROUGH OF THE YEAR
HIV Treatment as Prevention. Jon Cohen. Science 23 Dec. 2011 Vol.334, Issue 6063, pp. 1628
Microfabrication of human organ-on-chips: Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston Massachusetts, USA. Department of Bioengineering, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Viral Hepatitis Treatments: UCSF Health.